Background: Ibr is an established standard of care in CLL and is the only once-daily Bruton tyrosine kinase inhibitor with significant overall survival benefit in randomized phase 3 studies in first-line CLL (RESONATE-2; ECOG1912). The synergistic combination of Ibr + Ven (oral inhibitor of BCL2) has been shown to mobilize and clear CLL cells from multiple disease compartments leading to deep responses, providing a rationale to evaluate time-limited treatment (Jain et al. NEJM 2019). CAPTIVATE (PCYC-1142) is a multicenter phase 2 study (NCT02910583) of first-line Ibr + Ven with 2 cohorts: Minimal Residual Disease (MRD) and Fixed-Duration (FD). For both cohorts, patients (pts) received 3 cycles of Ibr lead-in followed by 12 cycles of combined Ibr + Ven. Pts in the MRD cohort were randomized by MRD status to placebo or further treatment. In the pre-randomization phase of the MRD cohort, Ibr + Ven resulted in high rates of undetectable MRD (uMRD) in both peripheral blood (PB; 75%) and bone marrow (BM; 72%), with concordant uMRD results in 93% of pts with matched samples (Tam, ASH 2019). We present primary results from the MRD-guided randomization phase of the MRD cohort, evaluating whether this regimen allows for treatment-free remission in the setting of uMRD.
Methods: Pts <70 years with previously untreated CLL/SLL requiring therapy received 3 cycles of Ibr lead-in followed by 12 cycles of Ibr + Ven (Ibr 420 mg/day PO; Ven ramp-up to 400 mg/day PO). Pts with Confirmed uMRD (defined as uMRD serially over ≥3 cycles, and in both PB and BM) after 12 cycles of Ibr + Ven were randomized 1:1 to receive double-blind treatment with placebo or Ibr; pts who did not meet the definition of Confirmed uMRD were randomized 1:1 to receive open-label treatment with Ibr or continued Ibr + Ven. Primary endpoint was 1-year DFS rate in the Confirmed uMRD pts randomized to placebo vs Ibr; DFS was defined as survival without progression or MRD relapse. Key secondary endpoints were rates of uMRD (<10-4 by 8-color flow cytometry), response per iwCLL, progression-free survival (PFS), and adverse events (AEs).
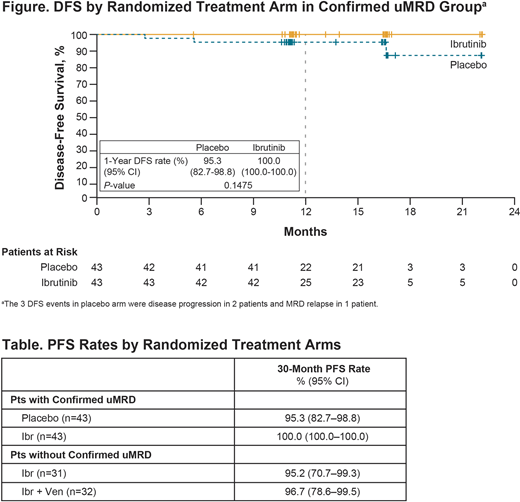
Results: 164 pts were enrolled in the MRD cohort. Median age was 58 years (range, 28-69); baseline high-risk features included del(17p) in 16% and del(11q) in 17%; del(17p) or TP53 mutation in 20%; complex karyotype in 19%; and unmutated IGHV in 60%. Median time on study was 31.3 mo (range, 15.0-41.0). 90% of pts completed planned treatment with 3 cycles of Ibr lead-in followed by 12 cycles of combined Ibr + Ven. Of 149 randomized patients, 86 (58%) with Confirmed uMRD (100% uMRD in PB and BM) were randomized to placebo (n=43) or Ibr (n=43). 63 of 149 pts (42%) did not achieve Confirmed uMRD as defined above and were randomized to Ibr (n=31) or Ibr + Ven (n=32); uMRD rates at randomization in this group were 48% in PB and 32% in BM. In the Confirmed uMRD group, 1-year DFS rate was not significantly different for pts randomized to placebo (95.3%; 95% CI 82.7-98.8) versus Ibr (100%; 95% CI 100-100) (P=0.1475; Figure). In the group without Confirmed uMRD who were randomized to continue Ibr or Ibr + Ven, uMRD rates improved to 57% in PB and 54% in BM during the overall study period. 30-mo PFS rates were >95% across all randomized arms (Table). Full results of endpoints by randomized arms will be presented. The median duration of treatment was 28.6 mo (range, 0.5-39.8) with Ibr and 12.0 mo (range, 0.8-34.1) with Ven. AEs were primarily grade 1/2 and mostly occurred in early cycles of Ibr + Ven, with modest differences by randomized treatment arm. During the overall study period across all-treated pts (with median treatment duration 29 mo), most common grade 3/4 AEs (≥5% of pts) were neutropenia (36%), hypertension (10%), thrombocytopenia (5%), and diarrhea (5%).
Conclusions: First-line Ibr + Ven treatment is an all-oral, once-daily, chemotherapy-free regimen that confers high rates of PB and BM uMRD in pts with CLL, and a 90% reduction in high-risk TLS monitoring (Siddiqi, EHA 2020). The 1-year DFS in pts randomized to placebo after Ibr + Ven combination was similar to that of pts continuing Ibr, supporting a fixed-duration treatment that offers treatment-free remissions in pts with CLL/SLL. The depth of response achieved with this regimen is reflected in the 30-mo PFS rate of ~95% across all treated pts. The safety profile of Ibr + Ven was consistent with known AEs for Ibr and Ven, and no new safety signals emerged.
Wierda:Genzyme Corporation: Consultancy; GlaxoSmithKline, Novartis, AbbVie, Genentech, Karyopharm, Pharmacyclics LLC, an AbbVie Company, Acerta Pharma, Gilead Sciences, Juno Therapeutics, KITE Pharma, Sunesis, Miragen, Oncternal Therapeutics, Cyclacel, Loxo Oncology, Janssen, and Xencor: Research Funding. Tam:BeiGene: Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria; AbbVie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Allan:AstraZeneca, Pharmacyclics LLC, an AbbVie Company, Genentech, AbbVie, Ascentage, and Cellectar: Consultancy; Celgene, Genentech, AstraZeneca, TG Therapeutics, and Janssen: Research Funding; Janssen, AbbVie, and AstraZeneca: Other: Travel/accommodations/expenses; Janssen, AstraZeneca, and AbbVie: Honoraria. Siddiqi:Pharmacyclics LLC, an AbbVie Company, Seattle Genetics, Janssen, and AstraZeneca: Speakers Bureau; PCYC: Membership on an entity's Board of Directors or advisory committees; Astrazenca: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics, Pharmacyclics LLC, an AbbVie Company, AstraZeneca, Celgene, Kite Pharma, and BeiGene: Consultancy; AstraZeneca: Other: Travel/accommodations/expenses; Pharmacyclics LLC, an AbbVie Company, Juno Therapeutics, KITE Pharma, AstraZeneca, TG Therapeutics, Celgene, Oncternal, and BeiGene: Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; BeiGene: Other: DMC member. Kipps:AbbVie, Celgene, Genentech-Roche, Gilead, and Pharmacyclics LLC, an AbbVie Company: Consultancy; AbbVie, Genentech-Roche, Oncternal, and Pharmacyclics LLC, an AbbVie Company: Research Funding. Opat:Amgen: Research Funding; Epizyme: Research Funding; Mundipharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Research Funding; AstraZenca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffman-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Tedeschi:BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen spa: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Department of Hematology Niguarda Hospital Milano: Current Employment; Sunesis: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Badoux:AbbVie: Honoraria, Other: Travel/accommodations/expenses. Kuss:Roche, Janssen, Gilead, and AbbVie: Speakers Bureau; Janssen, AbbVie, Roche, Mundipharma, Takeda, Merck, and Gilead: Consultancy, Honoraria. Jackson:AbbVie: Consultancy; Roche: Other: Travel/accommodations/expenses. Moreno:Janssen, AbbVie, Sunesis, and AstraZeneca: Consultancy; AbbVie and Janssen: Research Funding; Janssen: Speakers Bureau. Jacobs:AbbVie, Pharmacyclics LLC, an AbbVie Company, AstraZeneca, and Verastem: Consultancy; TG Therapeutics and Pharmacyclics LLC, an AbbVie Company: Research Funding; AbbVie, Pharmacyclics LLC, an AbbVie Company, AstraZeneca, Janssen, Sanofi, and Genentech: Speakers Bureau. Pagel:Gilead, Pharmacyclics LLC, an AbbVie Company, and AstraZeneca: Consultancy. Flinn:Curis: Research Funding; Curio Science: Consultancy; Calithera Biosciences: Research Funding; AstraZeneca: Consultancy, Research Funding; Nurix Therapeutics: Consultancy; Celgene: Research Funding; Constellation Pharmaceuticals: Research Funding; MorphoSys: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Iksuda Therapeutics: Consultancy; Gilead Sciences: Consultancy, Research Funding; Merck: Research Funding; Loxo: Research Funding; Forty Seven: Research Funding; Takeda: Consultancy, Research Funding; Karyopharm Therapeutics: Research Funding; Genentech, Inc.: Research Funding; Infinity Pharmaceuticals: Research Funding; Juno Therapeutics: Consultancy, Research Funding; Acerta Pharma: Research Funding; Janssen: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Triphase Research & Development Corp.: Research Funding; Verastem: Consultancy, Research Funding; Yingli Pharmaceuticals ≠: Consultancy, Research Funding; Rhizen Pharmaceuticals: Research Funding; Johnson & Johnson: Other; Roche: Consultancy, Research Funding; Vincera Pharma: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Portola Pharmaceuticals: Research Funding; Seattle Genetics: Consultancy, Research Funding; Teva: Research Funding; TG Therapeutics: Consultancy, Research Funding; Trillium Therapeutics: Research Funding; Pfizer: Research Funding; Great Point Partners: Consultancy; IGM Biosciences: Research Funding; F. Hoffmann-La Roche: Research Funding; Kite Pharma: Consultancy, Research Funding; ArQule: Research Funding; Incyte: Research Funding; Agios: Research Funding; Forma Therapeutics: Research Funding; Novartis: Research Funding; Unum Therapeutics: Consultancy, Research Funding. Zhou:AbbVie: Current equity holder in publicly-traded company; Pharmacyclics LLC, an AbbVie Company: Current Employment. Szafer-Glusman:Pharmacyclics LLC, an AbbVie Company: Current Employment. Ninomoto:AbbVie, Amgen, and Celgene: Current equity holder in publicly-traded company; AbbVie: Current Employment. Dean:AbbVie: Current equity holder in publicly-traded company; Pharmacyclics LLC, an AbbVie Company: Current Employment. James:Pharmacyclics LLC, an AbbVie Company: Current Employment, Other: Leadership, Patents & Royalties: and other intellectual property; AbbVie: Current equity holder in publicly-traded company. Ghia:ArQule: Consultancy, Honoraria; Acerta/AstraZeneca: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Adaptive, Dynamo: Consultancy, Honoraria; Novartis: Research Funding; Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Celgene/Juno: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Lilly: Consultancy, Honoraria; MEI: Consultancy, Honoraria; Sunesis: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding.
Ibrutinib in combination with venetoclax is not approved in any indication.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal